Study population
We retrospectively analyzed the case data of 699 patients (aged ≥ 65 years) who underwent radical surgery for esophageal cancer from January 1, 2014 to January 31, 2017 in the perioperative database of geriatric thoracic…
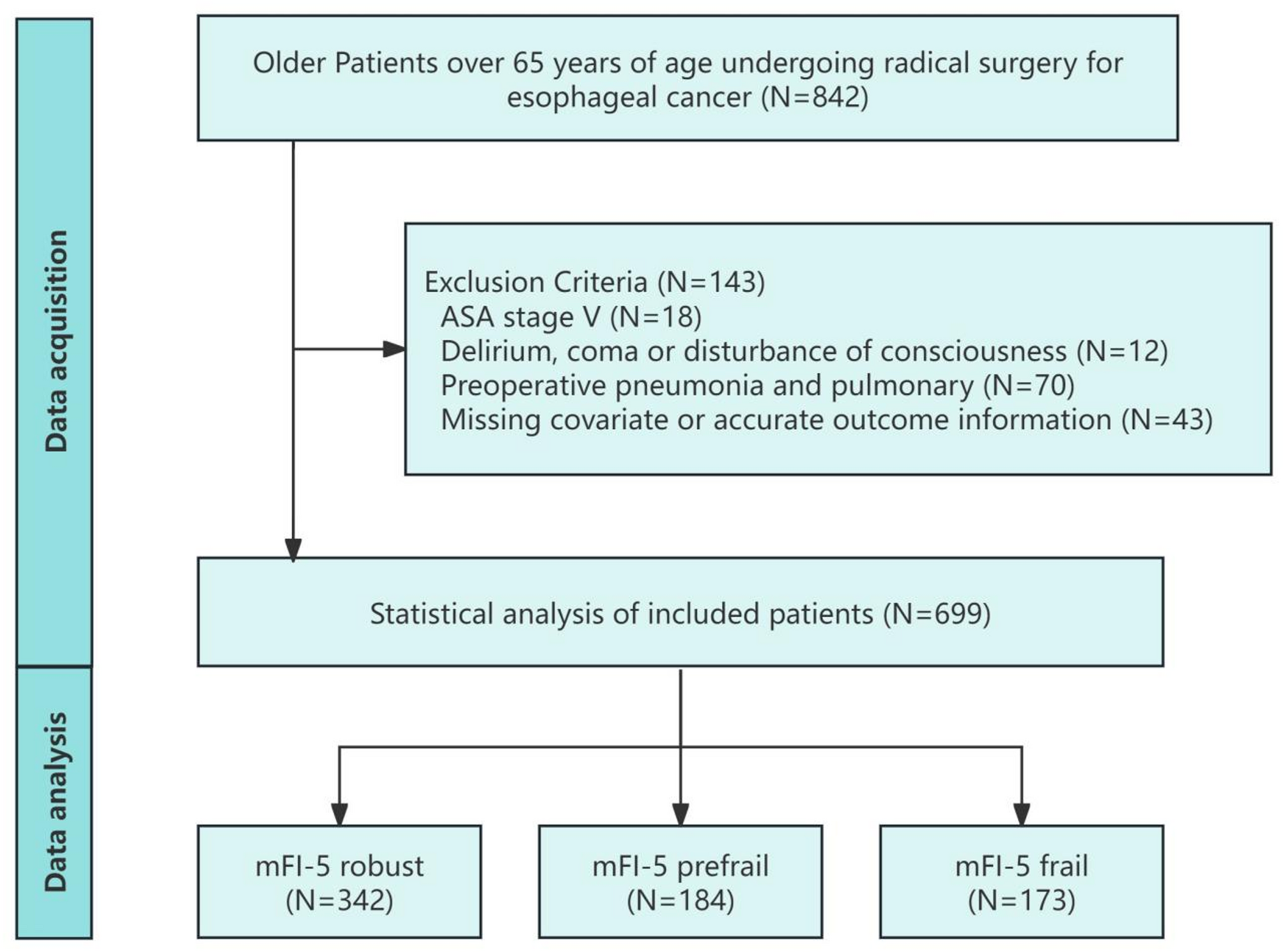
We retrospectively analyzed the case data of 699 patients (aged ≥ 65 years) who underwent radical surgery for esophageal cancer from January 1, 2014 to January 31, 2017 in the perioperative database of geriatric thoracic…

Pancreatic cancer remains one of the deadliest cancers, with limited effective treatment options and a generally poor prognosis. According to the American Cancer Society, pancreatic cancer accounts for about 3% of all cancers in the United States, yet it is responsible for approximately 8% of all cancer deaths. In 2025, an estimated 67,440 Americans (34,950 men and 32,490 women) will be diagnosed with pancreatic cancer, and about 51,980 deaths (27,050 men and 24,930 women) are expected. The average lifetime risk of developing pancreatic cancer is about 1 in 56 for men and 1 in 60 for women.
In this challenging landscape, novel immunotherapies are being explored to improve patient outcomes. One such investigational agent is mitazalimab, a monoclonal antibody targeting CD40, a key immune co-stimulatory receptor. Developed by Alligator Bioscience, mitazalimab is currently being studied in combination with chemotherapy in metastatic pancreatic cancer, showing promising early-phase results.
Monoclonal antibodies (mAbs) are laboratory-engineered proteins that mimic the immune system’s ability to recognize and bind to specific targets, such as proteins on the surface of cancer cells. By doing so, they can block signals that tumors use to grow, mark cancer cells for destruction by the immune system, or deliver toxic agents directly to the tumor.
CD40 is a receptor found on antigen-presenting cells (APCs), including dendritic cells, B cells, and macrophages. It plays a central role in regulating immune responses by promoting the activation of T cells. In cancer immunotherapy, stimulating CD40 can turn these APCs into potent activators of the immune system, helping to mount a strong antitumor response.
Mitazalimab is a next-generation monoclonal antibody developed by Alligator Bioscience. It belongs to a class of drugs designed to stimulate the immune system against cancer. Specifically, it targets CD40, a receptor expressed on antigen-presenting cells such as dendritic cells, B cells, and macrophages. By activating CD40, mitazalimab enhances the body’s ability to recognize and destroy cancer cells.
This drug is being investigated primarily in metastatic pancreatic cancer, a disease with historically poor outcomes and limited treatment options. Mitazalimab represents one of the most advanced CD40 agonists currently in clinical development.
CD40 plays a key role in immune system activation. When CD40 is stimulated, it helps dendritic cells “teach” T cells to recognize and attack tumors. Mitazalimab binds to CD40 and mimics the natural signals that activate this immune pathway, leading to:
In preclinical studies, it has shown potential to improve immune response not only alone but also when used in combination with chemotherapy, checkpoint inhibitors, or cancer vaccines. It may also help generate durable antitumor immunity that persists after treatment ends.
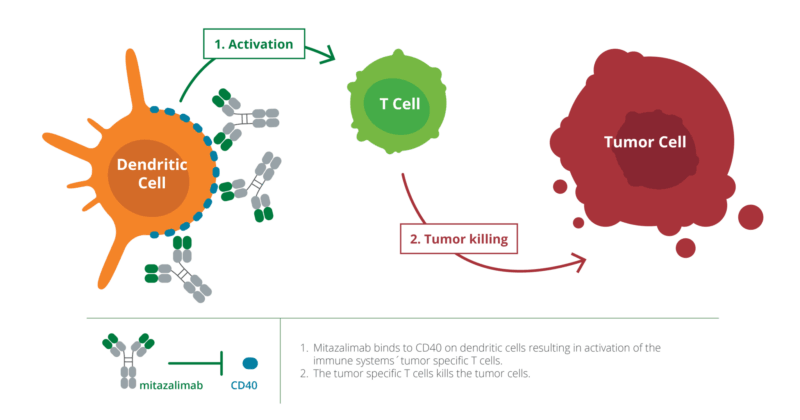
Photo from Alligator Bioscience Official Website.
Mitazalimab was initially evaluated in two Phase 1 trials. One, led by Alligator Bioscience, assessed intratumoral administration, while the other, conducted by Janssen Biotech, studied systemic administration in patients with advanced solid tumors. These trials confirmed mitazalimab’s favorable safety profile and demonstrated early signals of antitumor activity. One patient with renal cell carcinoma achieved a partial response, and ten patients experienced stable disease for six months or longer.
Mitazalimab’s mechanism of action was further supported by biomarker analyses. The drug activated key immune cells—including dendritic cells, macrophages, and T cells—enhancing both innate and adaptive immune responses. Transcriptomic profiling in patient-derived immune cells showed strong immune activation consistent with its CD40 agonist activity.
The REACTIVE-2 trial was a Phase 1, investigator-sponsored study evaluating mitazalimab in combination with the cancer vaccine MesoPher in patients with previously treated metastatic pancreatic cancer. The final patient was dosed in 2023. This study added further translational support to the immune-stimulatory potential of mitazalimab in this hard-to-treat malignancy.
The OPTIMIZE-1 study marked a major advancement in mitazalimab’s development. This open-label, multicenter Phase 2 trial evaluated mitazalimab in combination with mFOLFIRINOX in patients with previously untreated metastatic pancreatic ductal adenocarcinoma (PDAC).
At the 24-month cutoff:
At the data cutoff, 28% of patients remained alive, and 9% were still on active treatment. These results highlight mitazalimab’s potential to enhance the depth and durability of responses when combined with chemotherapy.
Previously published in The Lancet Oncology 2024, the OPTIMIZE-1 trial showed that among 57 evaluable patients with metastatic pancreatic ductal adenocarcinoma treated with mitazalimab plus modified FOLFIRINOX, the confirmed objective response rate was 40%, exceeding the primary endpoint. Median progression-free survival was 7.7 months, and median overall survival reached 14.3 months, with 59% of patients alive at 12 months. At data cutoff, 51% remained on study and 32% on treatment.
The median duration of response was 12.5 months. Treatment was generally manageable; the most common grade 3 or higher adverse events included neutropenia (26%), hypokalemia (16%), anemia, and thrombocytopenia (both 11%). Serious adverse events occurred in 41% of patients but none were attributed to mitazalimab. No treatment-related deaths were reported. These encouraging results support further phase 3 evaluation of this combination.
00263-8/asset/c1404d50-9e80-463d-9688-a16b7f125c81/main.assets/gr3.jpg)
An updated analysis published in Cell Reports Medicine 2025 provided extended follow-up and biomarker insights. At a median follow-up of 18.2 months, the confirmed ORR was 42.1 % (one complete and 24 partial responses), with an unconfirmed ORR of 54.4 % and a disease-control rate of 78.9 %. The median duration of response was 12.6 months, PFS 7.7 months, and OS 14.9 months, with 12- and 18-month OS rates of 57.8 % and 36.2 %, respectively.
Exploratory biomarker analyses identified a fibrosis-related gene signature enriched for extracellular-matrix-remodeling genes (MMP2, MMP14) associated with improved survival, and mitazalimab-induced activation of T, B, NK, and myeloid cells correlated with longer PFS and OS. Circulating-tumor-DNA analyses showed ctKRAS clearance in 72 % of patients, predicting longer survival; molecular responses preceded radiologic responses by ≈47 days, while molecular progression appeared ≈39 days earlier than imaging.
Based on FDA guidance, an expansion cohort of OPTIMIZE-1 tested a lower 450 µg/kg dose. Topline results showed an ORR of 22.7% at this dose—less effective than the 54.4% seen at 900 µg/kg—confirming the need to advance the higher dose into Phase 3.
Alligator Bioscience has completed CMC requirements, including GMP manufacturing readiness. Both the U.S. FDA and Germany’s Paul Ehrlich Institute confirmed that OPTIMIZE-1 qualifies as a Phase 3-enabling trial.
A final Phase 3 protocol was submitted at the FDA’s End-of-Phase-2 meeting in January 2025. Regulatory agencies have agreed that the proposed design is suitable to support a future Biologics License Application (BLA) and Marketing Authorization Application (MAA).
Alligator Bioscience is planning to launch a global Phase 3 trial in the second half of 2025, potentially positioning mitazalimab for accelerated approval in first-line metastatic pancreatic cancer.
La Roche-Posay has found that scarring and eczema take a “profound” psychological toll on people. Respondents to the Scars of Life Epidemiological Study reported diminished self-esteem, social stigmatization, and limited personal and social…

After a successful early career in karting, Garcia progressed through single-seater racing, competing in the Spanish F4 Championship in 2016 before joining the Renault Sport Academy in 2017.
She went on…

Terms
While we only use edited and approved content for Azthena
answers, it may on occasions provide incorrect responses.
Please confirm any data provided with the related suppliers or
…

Samsung Electronics (UK) Limited has announced it is partnering with Eco-Bos, to deliver pioneering smart home technology that drives down carbon emissions and increases energy efficiencies for the West Carclaze Garden Village in Cornwall. Residents in West Carclaze can enjoy lower energy bills and effortless control of their living environment, thanks to the Samsung SmartThings platform that lets them choose how to manage heating, lighting, security, and appliances all from a single app.
One of the UK’s first Garden Villages the 1,500 homes set within 500 acres, 350 which are a Country Park, in West Carclaze, places wellbeing and innovation at its core, providing homes that are highly energy efficient and designed to take full advantage of new technologies and innovative materials. The development also includes a 7.5 MW onsite solar farm to support renewable energy supply. Critically, West Carclaze homes are rated EPC A, a rating achieved by only 0.3% of homes in the UK in an area noted as one of the least energy efficient counties in the UK*.
West Carclaze residents will benefit from this pioneering development, with each household saving both time and money through the improvements. Community residents can expect to generate a profit of up to £1,779, based on SAP (Standard Assessment Procedure) calculations as outlined in the Predictive Energy Assessment from the Elmhurst Energy Report. Comparatively, a typical UK family household’s electricity and gas bill is approximately £1,719.45 per year, highlighting the potential for significant financial savings of up to £3,498 per year.[1]
As part of the collaboration, Samsung will provide its smart devices and appliances to each of the remaining 1,500 homes. Every property will come equipped as standard with a Samsung Heat Pump, Climate Hub, Solar PV as well as smart devices to handle everyday tasks across cooking, laundry, and dishwashing. All connected and managed in one simple interface via Samsung’s SmartThings[2] app, available on Android or iOS. The open ecosystem gives residents the ability to connect more than 350 partner brands within their home, including Amazon Alexa, Philips Hue and many other devices which be tailored based on each residents’ evolving needs.
Thanks to Samsung’s SmartThings platform and app, residents can seamlessly manage everyday tasks and benefit from SmartThings’ AI Energy Mode. This automatically switches appliances such as washing machines, fridge freezers and ovens to save energy through intelligent automation and learning capabilities within the appliances. For example a Samsung washing machine could reduce energy use by up to 20%[3] by simply switching on AI Energy Mode. By empowering residents to optimise their energy use and automate everyday routines, the tech at the heart of West Carclaze Garden Village will help create a more efficient community where residents can enjoy lower running costs of their home through reduced energy usage.
Mark Seaman, Head of Samsung B2B Integrated Offering Team, commented: “We know that people want homes that are more affordable to run and easier to live in. By partnering with Eco-Bos, we’re making it possible for residents to enjoy real savings on their energy bills, and giving them the opportunity to gain time back for themselves through the convenience and benefits of connected living.
“For developers, this collaboration shows that building tech-enabled communities is achievable today, meeting both new regulations and the needs of modern homeowners. We’re excited to help set a new standard for what home life can be, and we hope West Carclaze will inspire others to follow.”
Dorian Beresford, Chief Development Officer at Eco-Bos added: “Our collaboration with Samsung shows what’s possible when innovation and technology combine to serve people, not just performance. The future of housing must be cleaner, smarter, and more adaptable to people’s lives — with homes that work for people, not the other way around.
“This project brings that vision to life. Every home is designed to give back more than it takes — in energy, comfort, and quality of life. It’s proof that carbon-positive living isn’t a dream for tomorrow; it’s happening today.”
To learn more about Samsung’s work with homebuilders and developers, as well as its commitment to supporting the technological development of Tomorrow’s Homes Today please click here.
[*]*England’s Best and Worst Areas for Energy Efficient Homes – According to EPC data analysed by Eurocell
[1]British Gas, ‘What is the average energy bill in Great Britain?’, 2025.
[2]To use Samsung’s SmartThings service, you must have a Samsung Account, the SmartThings app installed on your smartphone or tablet, an active internet connection, and at least one compatible SmartThings-enabled device. The Samsung account is essential for storing your data and accessing your smart home setup across multiple devices, while the internet connection allows your devices to connect to the SmartThings network.
[3]Based on internal testing on the WW11BB944AGB model on a Cotton 40 degrees wash with the AI Energy Mode turned on (reducing the temperature) compared to not using AI Energy Mode. AI Energy Mode can only be operated at 40 degrees or lower.
Gestational Hypertension and Preeclampsia: ACOG practice bulletin summary, number 222 published by the American College of Obstetricians and Gynecologists (ACOG). Obstet Gynecol. 2020;135:1492-95.
Li Q, Zhao C, Liu M, Li M, Zhang Y, Yue C. Association…

On a recent Friday night in the vast atrium space of the Museum of Modern Art in New York, six string players took their place in a semi-circle and began performing the first movement of one of Mozart’s most sanctified sonatas. For the first…

BBC Asian Network has announced a new schedule kicking in from Monday 27 October.
Among the changes, Asian Network’s brand-new speech and current affairs programme, Asian Network Trending, will air every Wednesday, 8pm-10pm. The two-hour show…

Driverless taxis from Waymo will be available for hire on London’s roads next year, the US company has announced.
The UK capital will become the first European city to have an autonomous taxi service of the kind now familiar in San Francisco and four other US cities using Waymo’s technology.
Waymo said its cars were now on their way to London and would start driving on the capital’s streets in the coming weeks with “trained human specialists”, or safety drivers, behind the wheel.
The company – originally formed as a spin-off from Google’s self-driving car programme and part of the same parent group, Alphabet – said it would scale up operations and work closely with the Department for Transport and Transport for London to obtain the necessary permissions to offer fully autonomous rides in 2026.
Uber and the UK tech company Wayve have also announced their own plans to trial their driverless taxis in the capital next year, after the British government said it would accelerate rules allowing public trials to take place before legislation enabling self-driving vehicles passes in full.
The transport secretary, Heidi Alexander, said: “I’m delighted that Waymo intends to bring their services to London next year, under our proposed piloting scheme.
“Boosting the AV sector will increase accessible transport options alongside bringing jobs, investment and opportunities to the UK. Cutting-edge investment like this will help us deliver our mission to be world leaders in new technology and spearhead national renewal.”
A fuller rollout of self-driving taxis is expected in the UK after the Automated Vehicles Act fully takes effect in late 2027.
Waymo already has ties to Britain after opening its first European engineering hub in Oxford in 2019. It is also launching services in Tokyo using Jaguar Land Rover electric vehicles, its only other current venture outside the US.
after newsletter promotion
The US company’s co-chief executive Tekedra Mawakana said the technology was “making roads safer and transportation more accessible”, adding: “We’ve demonstrated how to responsibly scale fully autonomous ride-hailing, and we can’t wait to expand the benefits of our technology to the United Kingdom.”
Waymo launched its autonomous taxis in 2020 and now says it has taken more than 10 million passengers in the US.
Despite some alarming incidents, Waymo said the data showed that cars driven by humans were involved in incidents that injured pedestrians 12 times more often than its autonomous vehicles.